About Ice Cores Part II

Cross-section of an ice core. Photo: Sepp Kipfstuhl
In the first article about ice cores, we learned why ancient stories lie dormant in the ice. We got to the icy library and looked at how the stories can be recovered in the form of ice cores. This article will deal with the question how we can reveal the stories of the ice cores. To do this, we need to understand the different languages of the ice.
This is the second article of the series “About Ice Cores”. Here you can find the first article.
 The Studies of the Historical Books made of Ice
The Studies of the Historical Books made of Ice
Now we have brought all the chapters of the icy book to the surface. And now? Reading in the ice core is laborious. Some parts of the language have already been deciphered, but we can still hardly read anything at first glance. For that we need a translation. This is done by measuring instruments, for example. But we couldn’t take most of the measuring instruments with us to the glacier, they are in the laboratories at the institutes. So we take the ice core with us. We borrow a book from the library. But we won’t be able to bring the ice core back after we’ve read it, because we have to partially melt the ice to read it. That’s actually a bit different from borrowing.
 The Secret of the Air Bubbles
The Secret of the Air Bubbles
Our ice core actually speaks several languages at the same time, because the ice, which comes from hundreds of metres below the surface, not only consists of frozen water. There are also small air bubbles trapped in the ice. These air bubbles contain one of the languages. Because these air bubbles contain air that can be thousands of years old.

Air bubbles in a thin layer of ice. Photo: Sepp Kipfstuhl
How does that happen? Fresh snow is very loose. There is a lot of air between the filigree flakes. Over the years, the snow becomes more and more compacted and new layers of snow are added on top. The snow becomes firn (the compacted, coarse-grained snow). In the spaces between the ice grains there still is air.
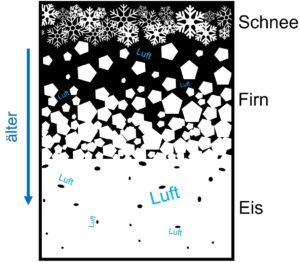
Schematic representation of how air (Luft) becomes trapped in the ice (Eis) with increasing time and depth (Schnee=snow, Firn=firn, älter=older). Illustration: Hanna Knahl
The firn grains move closer and closer together. They finally freeze together and the trapped air can no longer escape. No new air can flow in either. So each air bubble is a tiny little piece of atmosphere and represents the atmosphere at the time the air was trapped there. Without exaggerating, this is a fantastic thing! Because with these air bubbles we have the possibility to directly measure how the atmosphere was composed thousands of years ago. How much CO2 did it contain at times when it was particularly cold or particularly warm? How quickly did the CO2 concentration change? Was the proportion of oxygen in the atmosphere always the same? What about methane? The air bubbles thus contain the language that tells us the components of the atmosphere from the past.
To get a closer look at ice cores and their small air bubbles, we enter a giant freezer. In the following video from Terra X, we are taken into an ice laboratory at the Alfred Wegener Institute.
CC BY 4.0, ZDF/Terra X/Gruppe 5/Luise Wagner, Jonas Sichert
 Ice Can Store Temperatures
Ice Can Store Temperatures
The ice itself can also speak. It can tell us something about the temperature that prevailed when this ice formed. Sounds strange? Ice can’t just hold the temperature it had thousands of years ago. True! But the composition of the ice tells us something about the temperature.
Water – H2O – The H stands for hydrogen and the O for oxygen. There is “normal” oxygen and “heavy” oxygen. To understand the difference between the two, we need to understand how atoms are built.
Here you can find a brief description of atoms:
 Atoms and Isotopes
Atoms and Isotopes
The name atom comes from ancient Greek and means “indivisible”. When the name was given, it was assumed that atoms were the smallest possible particles. However, as scientists later discovered, atoms are not indivisible particles at all. Atoms consist of even smaller particles. These are…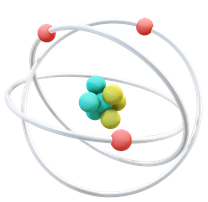
- … tiny and light electrons (red). These are arranged in different “shells” that surround the nucleus of the atom (represented for simplicity by the white rings). Electrons are electrically charged. They all have the same charge. This is defined as negative.
- … heavier protons (blue). They are much heavier than electrons. Their charge is the same as that of the electrons, but it is positive.
- … neutral neutrons (yellow). They are just as heavy as the protons, but have no electrical charge – they are neutral.
Protons and neutrons together form the nucleus of the atom. The weight of the atom depends on the number of protons and neutrons (the electrons are so light in comparison that we can neglect their weight). In other words, to determine the weight of an atom, we count protons and neutrons and multiply them by their mass (which is the same).
Most “classical” atoms have the same number of protons and neutrons in their nucleus. It depends on this number whether we assign the atom to the element “oxygen” (8 protons + 8 neutrons) or “silver” (47+47), for example.
However, the number of neutrons can also vary. Then we speak of different isotopes of the same element. To distinguish between them, we write the sum of the number of protons and neutrons in front of the element, e.g. 16O for the “classical” oxygen.
There is “normal” oxygen (16O), which has a nucleus consisting of 8 protons and the same number of neutrons as a “classical” atom. But there are also other stable forms of oxygen, such as “heavy” oxygen. This also has 8 protons, but 10 neutrons (i.e. 2 neutrons more). This oxygen is therefore heavier.
Water – H20 – can contain both the “normal” and the heavy oxygen. An interesting property of water with heavy oxygen, which we are now using, is that this heavier water also evaporates with more difficulty.
The ice on the ice sheet forms from the snow that has fallen on it. The snow comes from clouds and the water and ice in the clouds comes mainly from the oceans. The water from the ocean surface evaporates and rises. When it is cold, it is mainly the water with the lighter oxygen that evaporates. But the warmer it gets, the more the water with the heavier oxygen also evaporates. So when it’s warm, a lot of heavy oxygen also ends up on the ice. And if we find a lot of heavy oxygen in a layer of ice, then the snow probably fell during a warm climate period.

Water evaporates from the ocean, is transported in clouds and falls down on the ice sheet as snow flakes. Illustration: Hanna Knahl
 One important information is still missing
One important information is still missing
Ok, now we understand the language of the air bubbles and the temperature in the ice. This is super interesting and tells us a lot about the climate of the past. We can also have a look which composition of the atmosphere is related to which temperature. It’s really exciting! This way we can also learn something about our current climate and have clues about how the climate could develop in the future.
But something important is still missing. What we know so far: “At some point thousands of years ago, this temperature prevailed and the atmosphere was composed in this and that way”. Now, of course, we want to know: When exactly? How can we obtain this information from the ice?
This, we will find out in the last article (Part III) of the ice core series.
 Sources and Further Links
Sources and Further Links
The article series “About Ice Cores” is based on the podcast advents calendar “Eis hoch 24” (ice to the power of 24) from 2020 (in German).
Youtube: https://www.youtube.com/channel/UC8ZphUO3AC8Xbt4SwXd589A
The topics of this article are discussed in episode 4 (Klimarekonstruktion/climate reconstruction) & episode 11 (Was sind eigentlich Gletscher?/What are glaciers?).
Climate Reconstruction
https://www.iceandclimate.nbi.ku.dk/research/drill_analysing/
Atoms
Isotopes








0 Comments
2 Pingbacks