By Thorne Abend

Figure 1: Source: 2025, Idiogram AI – The Fight of Defense Peptides Against Bacteria
Infections caused by bacteria can become a serious problem for patients. Antibiotics are often prescribed in such cases. However, a group of small proteins now offers hope in the fight against bacterial infections. These proteins could help address bacterial colonies on implants.
Are antimicrobial peptides the new antibiotics?
One in 20 patients faces a very high risk of bacterial infection after undergoing surgical implantation [1]. In the UK alone, bacterial infections caused by urinary catheters are responsible for 2,100 deaths annually [2].
How can we win the fight against bacteria and reduce the risk of severe infections? The answer might lie in a small group of proteins known as peptides. These peptides, also called antimicrobial peptides (AMPs), are short proteins composed of 12 to 50 building blocks called amino acids. The type of building blocks determines the peptide’s function. AMPs are effective against a broad range of organisms, including bacteria, enveloped viruses, fungi, and even tumor cells. These peptides act like tiny knives, piercing the outer membrane of bacteria and killing them.
AMPs work due to two key properties: they are both water-attracting (hydrophilic) and fat-attracting (lipophilic), a characteristic referred to as amphipathic. This allows them to attach to the outer membrane of bacteria and penetrate it.
If AMPs act like tiny knives that invade and destroy cells, why aren’t they dangerous to humans? Their targeted action comes from the fact that these “knives” only become harmful when the cell membrane has a high negative charge, which strongly attracts the AMPs. Since bacterial membranes are much more negatively charged than mammalian cell membranes, human cells are not affected, and only bacteria are attacked.
The advantage of AMPs over antibiotics is that they are not limited to targeting a small group of bacteria or needing specific modifications to be effective; they work against a wide variety of bacteria. Additionally, unlike antibiotics, which often only inhibit bacterial growth, AMPs actively kill bacteria.
They typically target the membrane of pathogens (e.g., by disrupting cell membranes) or interfere with cellular processes in ways that make it harder for bacteria to mutate or adapt. This makes it more difficult for bacteria to develop resistance compared to antibiotics, which often target specific proteins or enzymes that can mutate over time.
AMPs can be used in combination with traditional antibiotics to enhance their effectiveness, reduce the required antibiotic dose (minimizing side effects), and delay or prevent the development of resistance. AMPs can also be engineered or modified to improve their stability, specificity, or activity against resistant strains, offering a customizable option for future therapies.
However, AMPs face challenges, including potential toxicity at high doses, difficulties in large-scale production, and stability issues. Low metabolic stability, which is an inherent risk of therapeutic peptides in general, is another key factor limiting their clinical application. Peptide-based drugs generally have low oral bioavailability due to pre-systemic enzymatic degradation and poor penetration of the intestinal mucosa, making oral administration typically impossible.
Nonetheless, ongoing research aims to overcome these hurdles and turn AMPs into a viable alternative or complement to antibiotics.
Antimicrobial peptides slice through bacteria like knives.
The mechanism of action of antimicrobial peptides (AMPs) is not yet fully understood. It is suspected that not all of them function in the same way. The three most common models are the barrel-stave model, the toroidal-pore model, and the carpet model. [4]
It is suspected that not all of them function in the same way. The three most common models are the barrel-stave model, the toroidal-pore model, and the carpet model. [4]
- The barrel-stave model assumes that antimicrobial peptides embed themselves in the bacterial membrane like staves in a barrel, creating a central pore. This disrupts the integrity of the membrane, ultimately leading to the death of the bacterium.
- The toroidal-pore model is similar to the barrel-stave model, but in this case, the antimicrobial peptides not only penetrate the membrane but also deform it into multiple small vesicles. This results in a fragmented, non-continuous membrane, destroying its integrity and causing the bacterium’s death.
- The carpet model is based on the hypothesis that antimicrobial peptides adhere to the bacterial membrane in a dense arrangement. This disrupts the membrane’s ability to function properly, ultimately leading to the bacterium’s death.
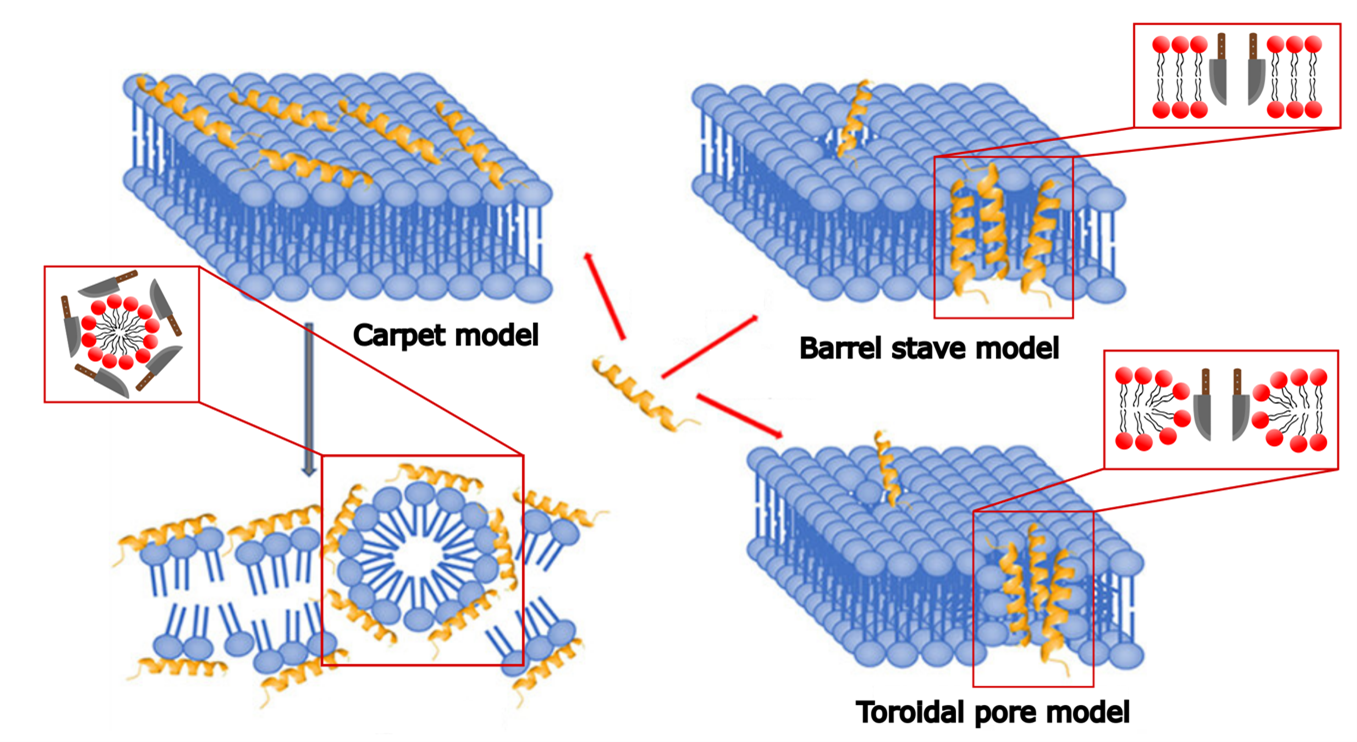 Figure 2: Models of the Mechanism of Action of Antimicrobial Peptides – Function Analogous to Knives. Barrel-Stave Model: The peptides penetrate the bacterial membrane, cutting into it and creating an opening. Toroidal-Pore Model: The peptides attach to the heads of the membrane molecules, forming an opening in the membrane. Carpet Model: The peptides arrange themselves like a carpet around the membrane molecules, breaking them down into many small fragments. Adapted from [4].
Figure 2: Models of the Mechanism of Action of Antimicrobial Peptides – Function Analogous to Knives. Barrel-Stave Model: The peptides penetrate the bacterial membrane, cutting into it and creating an opening. Toroidal-Pore Model: The peptides attach to the heads of the membrane molecules, forming an opening in the membrane. Carpet Model: The peptides arrange themselves like a carpet around the membrane molecules, breaking them down into many small fragments. Adapted from [4].
Probing with a Needle in Search of the Blueprint
Since antimicrobial peptides are composed of many different building blocks, each of which can slightly alter their function, they must be thoroughly researched. One challenge for researchers is finding ways to anchor antimicrobial peptides onto surfaces while preserving their activity. This could enable the coating of implants and catheters to prevent the growth of biofilms (bacterial colonies).
At the University of Bremen, researchers are tackling this anchoring challenge using an atomic force microscope. In the research group of Prof. Dr. Colombi Ciacchi, blueprints for antimicrobial peptides are being studied. [3] The atomic force microscope is equipped with a probing needle that is several orders of magnitude smaller than a single cell. Antimicrobial peptides can be attached to this needle. When the probe is placed on a bacterium, the interaction—measured as the adhesion forces of the antimicrobial peptide with the bacterium during detachment—can be analyzed.
For this process, the bacterium is kept in a buffer solution designed to mimic the body’s internal environment. This allows researchers to determine whether the blueprint of a particular antimicrobial peptide is suitable for targeting a bacterium or not.
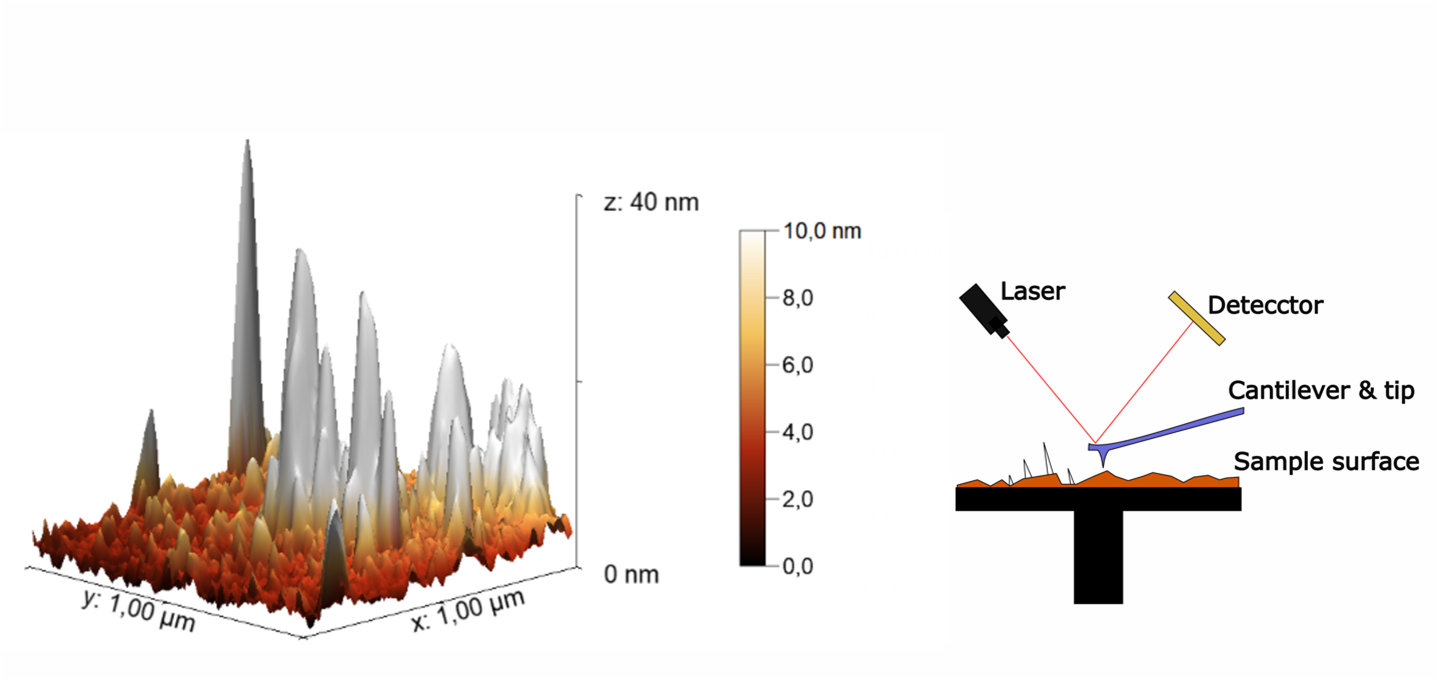
Figure 3: Imaging of Peptides Adsorbed onto a Surface Using an Atomic Force Microscope (AFM). The working principle of the AFM is shown on the right. The peptides appear as white protrusions on an otherwise macroscopically smooth surface.
A Promising Tool for Medicine
In summary, antimicrobial peptides have the potential to be effective against a wide range of bacteria. Like knives, they pierce the outer membrane of bacteria and kill them, while remaining harmless to human cells. The interaction of various antimicrobial peptides with bacteria can be studied using an atomic force microscope, allowing the development of the most effective blueprint for application.
Currently, more and more of these peptides are being discovered in plants, insects, and other animals, with their potential still largely unexplored. [3] Overall, antimicrobial peptides represent a promising tool for medicine that could soon be used to combat bacterial infections associated with implants and catheters.
Has this topic piqued your interest? – Learn more in the following articles:
- Zhang, QY., Yan, ZB., Meng, YM. et al.Antimicrobial peptides: mechanism of action, activity and clinical potential. Military Med Res 8, 48 (2021). https://doi.org/10.1186/s40779-021-00343-2.
- Bahar AA, Ren D. Antimicrobial Peptides. Pharmaceuticals. 2013; 6(12):1543-1575. https://doi.org/10.3390/ph6121543.
- V.R. Reddy, R.D. Yedery, C. Aranha, Antimicrobial peptides: premises and promises, International Journal of Antimicrobial Agents, Volume 24, Issue 6, 2004, Pages 536-547, ISSN 0924-8579, https://doi.org/10.1016/j.ijantimicag.2004.09.005.
Literature
[1] Anderson, J. M. und Marchant, R. E. (2000). Biomaterials: factors favoring colonization and infection. Infections associated with indwelling medical devices,Seiten 89–109. http://dx.doi.org/10.1128/9781555818067.ch5
[2] Feneley, R. C., Hopley, I. B., und Wells, P. N. (2015). Urinary catheters: history, cur- rent status, adverse events and research agenda. Journal of medical engineering & technology, 39(8):459–470. https://doi.org/10.3109/03091902.2015.1085600
[3] Corrales-Ureña YR, Souza-Schiaber Z, Lisboa-Filho PN, Marquenet F, Michael Noeske PL, Gätjen L, Rischka K. Functionalization of hydrophobic surfaces with antimicrobial peptides immobilized on a bio-interfactant layer. RSC Adv. 2020 Jan 2;10(1):376-386. doi: 10.1039/c9ra07380a.
[4] Huang, X., & Li, G. (2023). Antimicrobial peptides and cell-penetrating peptides: non-antibiotic membrane-targeting strategies against bacterial infections. Infection and Drug Resistance, 1203-1219. http://dx.doi.org/10.2147/IDR.S396566








Leave a Reply